This article will take a deep dive into powder metallurgy
You will learn about the following 5 topics
1. What is powder metallurgy
2. Powder metallurgy process
3. Aerospace parts and products made from powder metallurgy
4. Metal materials used in powder metallurgy
5. More...
What is powder metallurgy?
Powder metallurgy is an industry that manufactures metal powders. It uses metal powders (including a small amount of non-metallic powders mixed in) as raw materials to manufacture materials and products through a molding-sintering process. With the development of modern powder metallurgy manufacturing technology, powder metallurgy products have replaced conventional metal casting, forging, cutting and mechanical parts with complex structures and difficult cutting, and their supporting application areas are constantly expanding.

From general machinery manufacturing to precision instruments, from hardware tools to large machinery, from the electronics industry to motor manufacturing, from civil industry to military industry, from general technology to cutting-edge high technology, powder metallurgy technology can be seen. In the field of civil industry, powder metallurgy products have become indispensable supporting basic parts for industries such as automobiles, motorcycles, home appliances, power tools, agricultural machinery, and office appliances.
The huge market potential also drives the advancement of technology. With the continuous expansion of the application scope of powder metallurgy products, the requirements for the size, shape, and performance of metal powder particles are getting higher and higher, and the performance, size, and shape of metal powders largely depend on the production method and preparation process of the powders. Therefore, the preparation technology of powders is also constantly developing and innovating.
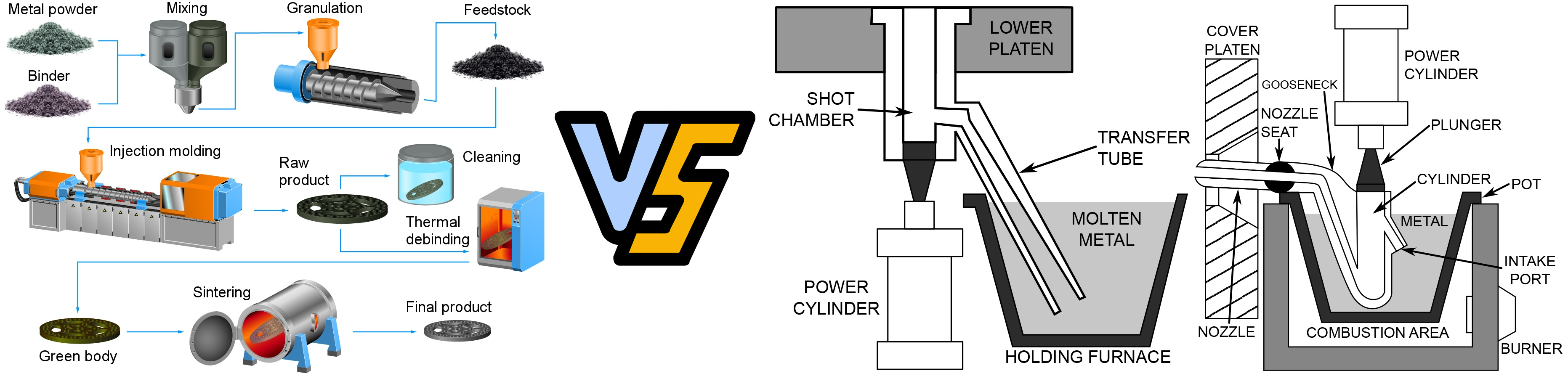
Traditional Powder Metallurgy Process Steps
-
Powder preparation → Mixing → Pressing → Sintering → Post-processing →
Inspection and packaging
1. Powder Preparation
- Purpose: Produce metal or alloy powders that meet specific requirements for particle size, shape, and purity.
-
Methods:
- Physical Methods: Atomization (gas atomization, water atomization), mechanical milling.
- Chemical Methods: Reduction, electrolysis.
- Mechanical Alloying: Blending metal or non-metal powders through ball milling.
- Key Control Points: Powder purity, particle size distribution, and oxidation levels.
2. Mixing
- Purpose: Homogeneously mix metal powders with lubricants, binders, or alloying components.
-
Methods:
- Dry Mixing: For simple compositions.
- Wet Mixing: Adds liquid agents to improve uniformity.
- Key Control Points: Ensure uniformity and avoid segregation of components.

3. Pressing and Shaping
- Purpose: Compact the powder into a desired shape, forming a green part.
-
Methods:
- Uniaxial Pressing: Common for simple shapes.
- Isostatic Pressing: Used for complex shapes or high-density requirements.
- Key Control Points: Control pressing pressure and green density to avoid defects like cracks or delamination.
4. Sintering
- Purpose: Heat the green part to create metallurgical bonding between powder particles, enhancing strength and density.
-
Processes:
- Solid-State Sintering: Heated below the melting point, particles bond via diffusion.
- Liquid-Phase Sintering: Some components melt to form a liquid phase, aiding particle bonding.
- Key Control Points: Sintering temperature, duration, and atmosphere (e.g., vacuum, inert gas).
5. Post-Processing (Optional)
Depending on product requirements, additional steps may include:
- Machining: Turning, milling, or grinding for precision dimensions and surface quality.
- Heat Treatment: Enhance mechanical properties.
- Surface Treatment: Electroplating, oxidation, or coating for corrosion resistance or aesthetics.
- Impregnation: Filling pores with oil or polymers to improve airtightness or lubrication.
6. Inspection and Packaging
- Purpose: Ensure finished products meet quality standards and customer specifications.
-
Inspection Items:
- Dimensional accuracy.
- Density and porosity.
- Mechanical properties, such as hardness and strength.
- Surface quality inspection.
- Packaging: Prevent oxidation or damage during transportation and storage.
Metal Materials Used in Aerospace Powder Metallurgy
1. Titanium and Titanium Alloys
-
Characteristics:
- High specific strength (excellent strength-to-weight ratio).
- Exceptional corrosion resistance and fatigue performance.
- Good high-temperature resistance.
- Common Alloys: Ti-6Al-4V, Ti-48Al-2Cr-2Nb (γ-TiAl).
-
Applications:
- Turbine engine blades, connectors, and fuselage structural components.
2. Superalloys
-
Characteristics:
- Excellent high-temperature strength and creep resistance.
- Outstanding oxidation and corrosion resistance.
-
Common Types:
- Nickel-based alloys (e.g., Inconel 718, Rene 95).
- Cobalt-based alloys (e.g., Haynes 188).
- Iron-based alloys.
-
Applications:
- Turbine blades, combustion chamber parts, rocket engine nozzles.
3. Aluminum and Aluminum Alloys
-
Characteristics:
- Low density and lightweight.
- Good machinability and corrosion resistance.
- Common Alloys: 7075 aluminum alloy, Al-Si alloy.
-
Applications:
- Aerospace structural components, support frames.
4. Molybdenum and Molybdenum Alloys
-
Characteristics:
- High melting point and excellent high-temperature performance.
- Good thermal conductivity and thermal shock resistance.
-
Applications:
- Spacecraft thermal barrier materials, rocket nozzles, electronic components.
5. Tungsten and Tungsten Alloys
-
Characteristics:
- Extremely high melting point and density.
- Excellent high-temperature resistance and radiation shielding properties.
-
Applications:
- Rocket nozzles, radiation shielding components, counterweights.
6. Iron-Based Powder Metallurgy Materials
-
Characteristics:
- Good mechanical properties and cost-effectiveness.
- Strength and wear resistance can be enhanced through alloying.
-
Applications:
- Gears, bearings, and support components.
Metal Materials Used in Powder Metallurgy
Although there are virtually no restrictions on the metals used in powder metallurgy, certain metals are preferred due to their specific properties and characteristics. Manufacturers evaluate various factors when selecting metals to meet their needs.

Key factors in choosing metals for powder metallurgy include corrosion resistance, hardness, tensile strength, impact toughness, and fatigue strength. Each metal possesses some or all of these properties, and the choice depends on the specific requirements of the components being produced.
1. Titanium
-
Characteristics:
- High specific strength (excellent strength-to-weight ratio).
- Exceptional corrosion resistance and fatigue performance.
- Good high-temperature resistance.
- Common Alloys: Ti-6Al-4V, Ti-48Al-2Cr-2Nb (γ-TiAl).
-
Applications:
- Turbine engine blades, connectors, and fuselage structural components.
2. Stainless Steel
-
Characteristics:
- Outstanding corrosion and rust resistance.
- Exceptional versatility and adaptability for various applications.
- Common Series: 300 and 400 stainless steels.
-
Notable Alloy:
- 316L Stainless Steel: Known for its excellent corrosion resistance, toughness, ductility, and acid resistance.
-
Applications:
- Aerospace components, automotive parts, medical instruments, and marine applications.
3. Copper and Copper Alloys
-
Characteristics:
- Excellent corrosion and rust resistance, particularly in humid environments.
- Can be used as pre-alloyed powders or elemental mixtures.
-
Common Alloy:
- Bronze: A copper-tin alloy commonly used for self-lubricating bearings.
-
Applications:
- Electrical components, bearings, and decorative parts.
4. Aluminum and Aluminum Alloys
-
Characteristics:
- Low density and lightweight.
- Excellent machinability and corrosion resistance.
- Common Alloys: 7075 aluminum alloy, Al-Si alloy.
-
Applications:
- Aerospace structural components and support frames.
5. Iron-Based PM Materials
-
Characteristics:
- Iron is a gray-black crystalline material with a density of 7.694 g/cm³ and a melting point of 1837°C.
- It is typically processed using high-pressure water atomization and sintered at a temperature of 1121°C.
- Due to its softness, iron is often alloyed with carbon to produce steel.
-
Applications:
- Automotive components such as gears, bearings, and support structures.
As a premium metal parts supplier specializing in metal powder metallurgy, we invite you to visit our website to learn more about our services. We are committed to providing customized aerospace parts solutions to promote innovative manufacturing of high-precision aviation equipment and spare parts. We look forward to working with you, please click the 【Request a Quote】link below Contact Us

Share:
Micro Metal Injection Molding Precision Solutions for Small Parts
What is Infiltration Powder Metallurgy